BsaI, GMP grade (GMP-BSA-EE101), DMF #037503
Catalog: GMP-BSA-EE101
Order now get it on
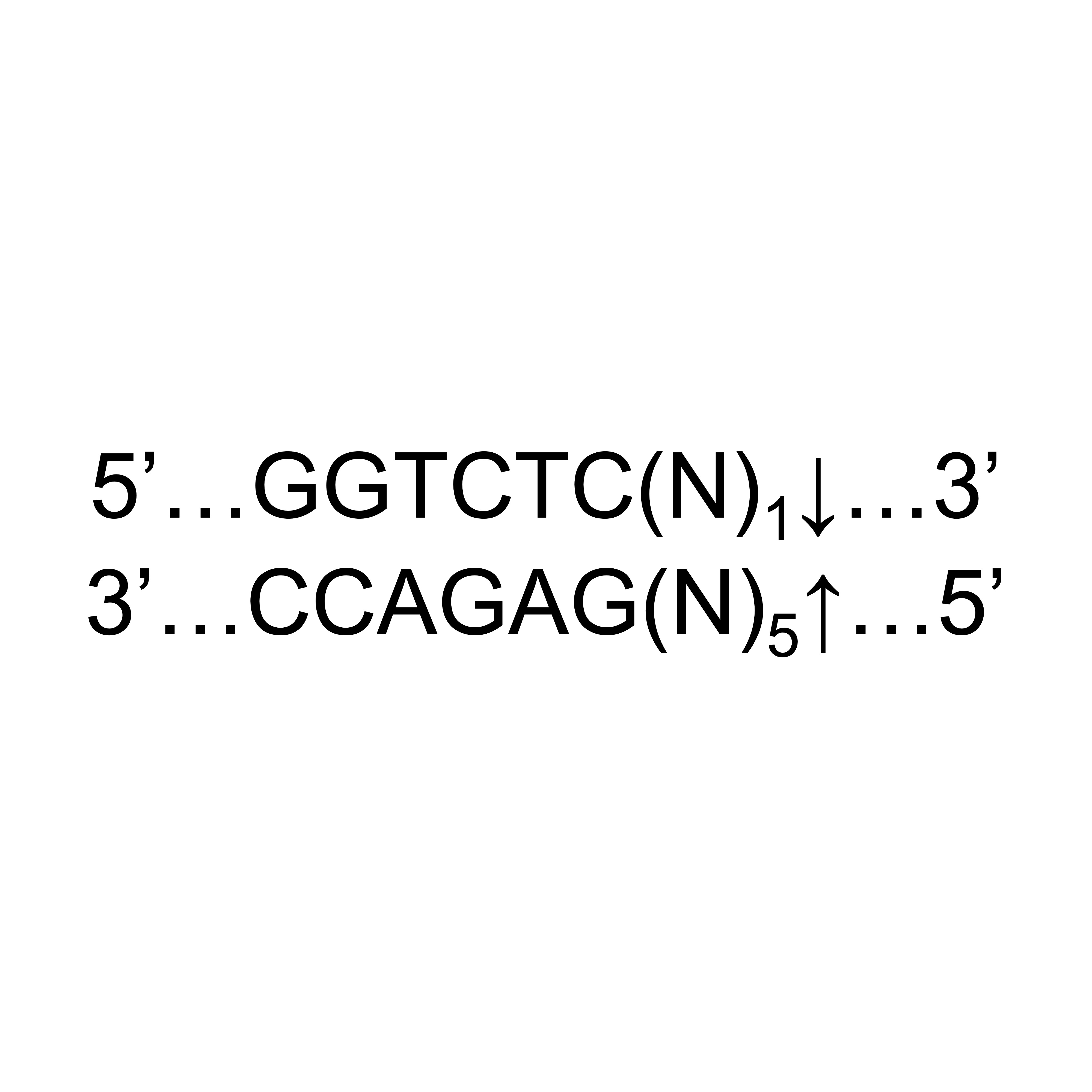
| Product Description | BsaI is designed to be used upstream of the in vitro mRNA synthesis step during mRNA therapeutics and vaccine manufacturing. As one of the type IIS restriction enzymes, it recognizes asymmetric DNA sequences and cleaves outside of their recognition sequence. The cleavage site of BsaI is GGTCTC (N1/N5), where the GGTCTC acts as the recognition site, and the N1/N5 represents the cleavage site with a 5' overhang. Kactus provides a BSA-free reaction buffer to ensure the safety of mRNA vaccine production. This product has been filed with the FDA Drug Master Files (DMF) and is assigned DMF #037503. |
| Synonyms | Bsa1 |
| Application | Genotyping SNP Plasmid linearization |
| Concentration | 20U/µL |
| Unit Definition | One unit is defined as the amount of enzyme required to digest 1 µg of pXba DNA in 1 hour at 37°C in a total reaction volume of 50 µL. |
| Source | Expressed in an E.coli strain that carries the BsaI gene from Bacillus stearothermophilus. |
| Quality Standards | Activity (Digestion activity detection): ≥ 20 kU/mL
Purity (SEC-HPLC): ≥ 95% Residual Protease: Negative Endotoxin: ≤ 2 EU/mL Residual DNase: Negative Residual RNase: Negative Residual Host Protein: ≤ 20 ng/mg Residual Host Cell DNA: ≤ 100 pg/mg Residual Heavy Metal: ≤ 10 ppm Residual Nickel Salt: ≤ 10 ppm Bioburden: ≤ 1 CFU/10mL |
| Form | Liquid |
| Shipping | Shipping with dry ice |
| Stability And Storage | -20±5°C for 12 months. Avoid repeated freeze-thaw cycles. |
You may also be interested in
View more associated products